NASF/AESF Foundation Research Project #122: Electrochemical Approaches to Treatment of PFAS in Plating Wastewater - 3rd Quarterly Report
The NASF-AESF Foundation Research Board has selected a project on addressing the problem of PFAS and related chemicals in plating wastewater streams, studying PFAS destruction via electrooxidation and electrocoagulation. This third quarter report continues the work on electrocoagulation, characterizing the floc morphology generated from plating wastewater, varying conditions in the electro-oxidation solution.
#pollutioncontrol #nasf
by
Qingguo (Jack) Huang*
College of Agricultural and Environmental Science
University of Georgia - Griffin Campus
Griffin, Georgia, USA
Editor’s Note: For 2021, NASF-AESF Foundation Research Board has selected a project on addressing the problem of PFAS and related chemicals in plating wastewater streams. This report covers the third quarter of work (July-September 2021). A printable PDF version of this report is available by clicking HERE.
Featured Content
Introduction
This project started in January 2021 with the goal of developing applicable electrochemical approaches to remove per- and polyfluoroalkyl substances (PFASs) present in plating wastewaters, including electrooxidation (EO) and electrocoagulation (EC). This project includes three research tasks that are designed to investigate EC, EO and EC-EO treatment train, respectively, designed to probe three hypotheses specified follows:
- EC generates amorphous metal hydroxide flocs that can effectively adsorb PFASs in plating wastewater, which, through an appropriate treatment, can release PFASs into a concentrated solution.
- EO enabled by a Magnéli phase Ti4O7 anode can be used to effectively destruct PFASs in plating wastewater.
- The electrochemical treatment train comprised of EC and EO by Ti4O7 anode can remove and degrade PFASs in plating wastewater more efficiently than either process operated individually.
This report describes our continuing effort in Task 1 by analyzing the floc morphology characterization during EC treatment, as well as the experiments of Task 3.
As extremely recalcitrant chemicals, the destruction of PFASs for treatment purposes is challenging for the conventional treatment technologies or advanced oxidation processes (AOPs) based on the hydroxyl radical (•OH).1 EO exhibits stronger degradation ability than conventional AOPs due to the combination of the direct electron transfer (DET) reaction on the anode and the oxidation by •OH produced by anodic oxidation of water,2 which has been proven effective in destroying PFASs using “non-active” anodes, including boron-doped diamond (BDD), PbO2, SnO2-Sb and Magnéli phase titanium suboxides.1,3-6 In particular, Magnéli phase titanium suboxides, with the general formula of TinO2n-1 (4 < n < 10), have recently been explored as a promising electrode due to their wide electrochemical window for water oxidation, chemical inertness and low production cost.7-9 The degradation of organic contaminants occurs only near or at the anode surface, which is usually limited by mass transfer from bulk solution to the anode surface.10 Enhancement of the target concentration in the bulk solution is an effective way to improve the EO treatment efficiency,11 and it appears that the EC process may well serve such a purpose.12,13
Experimental
An EC treatment was first carried out on a 250-mL solution of 10 PFASs (0.5 or 0.005 µM each) under 5 or 0.3 mA/cm2. The floc recovered at the end of treatment was dissolved with 4.5M H2SO4 solution and then brought up to 10 mL by Milli-Q water. The collected foam was also added with drops of the acid until the foam disappeared, and then brought up to 10 mL by Milli-Q water. The acid solution resulting from PFAS-laden flocs was directly subjected to EO treatment described below. The acid solution from the foam dilution was supplemented with 20 mM Na2SO4 as supporting electrolytes and then subjected to EO treatment.
EO experiments were conducted in a 25 mL electrolytic cell with a Ti4O7 ceramic plate (1 × 2 cm) of 3-mm thickness as the anode, and a 304 stainless steel rod (5 mm diameter) as the cathode that was placed in parallel to the central axis of the anode at a 2 cm gap. For the treatment, a 10 mL solution was placed in the cell with continuous stirring using a magnetic stirrer (IKA-RCT, Germany), while a direct current was applied to the electrodes with different current densities. For the floc dissolved acidic solution, in pre-specified time intervals, triplicate 0.1-mL samples were withdrawn, diluted with water and adjusted to a final pH of approximately 6.0, then added with 40 μL of a mixed isotope labeled internal standard solution (100 ppb), followed by further cleanup using solid phase extraction (SPE). For the foam solution, 0.1-mL samples were withdrawn and mixed with 0.1-mL isotope labeled internal standards for subsequent analysis of PFASs concentrations as described below. All EC and EO experiments were carried out at room temperature (25±1 ºC).
Results and Discussion
Flocs were formed during the EC treatment, and their appearance varied with current density and reaction time. The flocs generated in selected tests were collected by membrane filtration as described in the second report and then freeze-dried. The zinc hydroxide flocs samples were characterized for the morphology by Scanning Electron Microscope (SEM) on a Hitachi’s-4800 FE-SEM system (Hitachi, Japan), and for the Brunauer–Emmett–Teller (BET) surface area using a surface area analyzer (TriStar II Plus, Georgia). As shown in Fig. 1, the hydroxide flocs generated at low current density (0.3 mA/cm2) showed a more crystalline flake-like structure. When a higher current density (5 mA/cm2) was applied, the flocs exhibited a more clustered amorphous or loose structure. The BET surface areas of dried flocs after degassing for 4 hours were determined at 50°C.14 The BET surface areas of the dried flocs generated at low current density (0.3 mA/cm2) and high current density (5 mA/cm2) were 101.18 and 13.36 m2/g, respectively. Despite the lower specific surface of the flocs produced at the higher current density, the total quantity of flocs formed at the high current density was however much larger, resulting in higher PFAS removal. The floc quantities generated at 5 mA/cm2 was 16.67 times the quantities of floc generated using 0.3 mA/cm2 at the same reaction time.
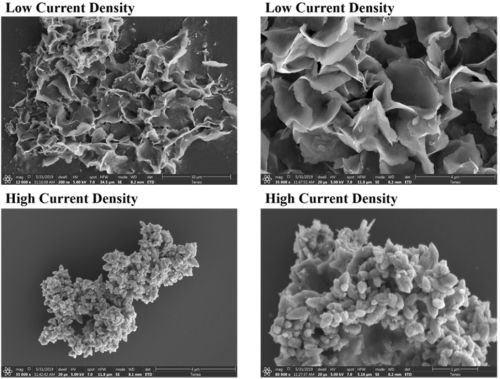
Figure 1 - Field emission SEM analyses of hydroxide flocs generated in-situ in the EC process using low current density (0.3 mA/cm2, 120 min) and high current density (5 mA/cm2, 60 min), respectively.
Three concentrated solutions prepared through the EC process were subjected to EO treatment using a Magnéli phase Ti4O7 anode at the current density of 10 mA/cm2. Solution I was the dissolved solution of PFAS-laden floc generated using a low current density condition after 120 min (0.3 mA/cm2, 0.005 µM each of 10 PFASs). Solution II was the acid dissolved PFAS-laden floc solution obtained through EC treatment under the high current density condition after 60 min (5 mA/cm2, 0.5 µM each of 10 PFASs). The foam collected during this EC process was supplemented with 20 mM Na2SO4 to a final volume of 10 mL as Solution III. The conductivity of Solution I and Solution II were 3.1 mS/cm and 20.8 mS/cm, respectively.
Figure 2 shows the concentration profiles of each PFAS in Solution I (Fig.2A), Solution II (Fig.2B) and Solution III (Fig.2C) during the EO treatments. PFAS removal in all three EC-derived solutions was evident during EO. Over 95% degradation of all PFASs was achieved within 60 min in Solution I except for PFBS (73.4%). The degradation of PFASs at 60 min in Solution III was over 90% except for PFBS (52.3%), PFHxA (74.2%) and 4:2 FtS (85.9%). This is consistent with other studies showing shorter chain PFASs are more recalcitrant to EO.15 All five PFASs in Solution II were removed over 90% within 60 min. PFAS degradation appeared to follow a pseudo-first order rate model. Similar degradation profiles were also found in our earlier study,16 which has verified that rapid mineralization to CO2 and F- was the main degradation pathway due to the DET reaction and surface-bound hydroxyl radical working in concert.5,6,16 Moreover, it should be noted that Solution I and Solution II had high conductivity because of the presence of zinc and sulfate ions, and therefore no supplement of electrolyte addition was needed, while 20 mM Na2SO4 was added to Solution III for the EO treatment. The energy efficiency of PFAS degradation was evaluated by EE/O that is defined as the electrical energy required to reduce the concentration of a pollutant by one order of magnitude (kWh/m3).
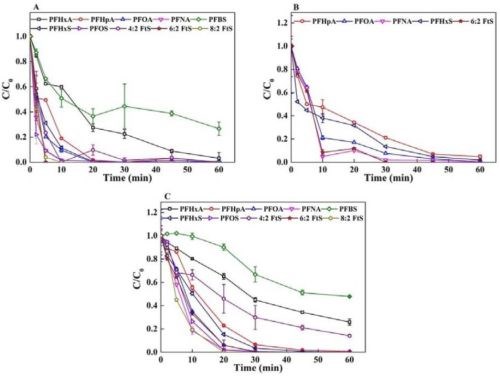
Figure 2 - PFAS concentration during EO treatment (current density =10 mA/cm2) of three solutions prepared by EC: (A) Solution I: acid dissolved solution of the PFAS-laden flocs produced by EC at 0.3 mA/cm2; (B) Solution II: acid dissolved solution of PFAS-laden flocs produced by EC at 5 mA/cm2; (C) Solution III: foam solution produced during EC at 5 mA/cm2.
For research purposes, the flocs and foams generated from the EC treatment at the high current density (5 mA/cm2, 0.5 µM each of 10 PFASs) were collected respectively in Solution II and III that were then separately subjected to the subsequent EO treatment. In practice, the flocs and foams can be combined in one solution for EO treatment.
References
1. K.E. Carter & J. Farrell, “Oxidative destruction of perfluorooctane sulfonate using boron-doped diamond film electrodes,” Environ. Sci. Technol., 42 (16), 6111-6115 (2008).
2. J-F. Zhi, H-B. Wang, T. Nakashima, T.N. Rao & A. Fujishima, “Electrochemical incineration of organic pollutants on boron-doped diamond electrode: Evidence for direct electrochemical oxidation pathway,” J. Phys. Chem. B, 107 (48), 13389-13395 (2003).
3. H. Lin, J. Niu, S. Ding & L. Zhang, “Electrochemical degradation of perfluorooctanoic acid (PFOA) by Ti/SnO2-Sb, Ti/SnO2-Sb/PbO2 and Ti/SnO2-Sb/MnO2 anodes,” Water Res., 46 (7), 2281-2289 (2012).
4. J. Niu, H. Lin, J. Xu, H. Wu & Y. Li, “Electrochemical mineralization of perfluorocarboxylic acids (PFCAs) by Ce-doped modified porous nanocrystalline PbO2 film electrode,” Environ. Sci. Technol., 46 (18), 10191 (2012).
5. H. Shi, Y. Wang, C. Li, R. Pierce, S. Gao & Q. Huang, “Degradation of perfluorooctanesulfonate by reactive electrochemical membrane composed of Magnéli phase titanium suboxide,” Environ. Sci. Technol., 53 (24), 14528-14537 (2019).
6. L. Wang, J. Lu, L. Li, Y. Wang & Q. Huang, “Effects of chloride on electrochemical degradation of perfluorooctanesulfonate by Magnéli phase Ti4O7 and boron doped diamond anodes,” Water Res.. 170, Article No. 115254 (2020).
7. F. Walsh & R. Wills, “The continuing development of Magnéli phase titanium sub-oxides and Ebonex® electrodes,” Electrochim. Acta, 55 (22), 6342-6351 (2010).
8. H. Lin, J. Niu, S. Liang, C. Wang, Y. Wang, F. Jin, Q. Luo & Q. Huang, “Development of macroporous magnéli phase Ti4O7 ceramic materials: As an efficient anode for mineralization of poly- and perfluoroalkyl substances,” Chem. Eng. J., 354, 1058-1067 (2018).
9. J. Radjenovic & D.L. Sedlak, “Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water,” Environ. Sci. Technol., 49 (19), 11292-11302 (2015).
10. C. Trellu, B. Chaplin, C. Coetsier, R. Esmilaire, S. Cerneaux, C. Causserand & M. Cretin, “Electro-oxidation of organic pollutants by reactive electrochemical membranes,” Chemosphere, 208, 159-175 (2018).
11. Á. Soriano, D. Gorri, L. Biegler & A. Urtiaga, “An optimization model for the treatment of perfluorocarboxylic acids considering membrane preconcentration and BDD electrooxidation,” Water Res., 164, Article No. 114954 (2019).
12. H. Lin, Y. Wang, J. Niu, Z. Yue, & Q. Huang, “Efficient sorption and removal of perfluoroalkyl acids (PFAAs) from aqueous solution by metal hydroxides generated in situ by electrocoagulation,” Environ. Sci. Technol., 49 (17), 10562-10569 (2015).
13. C. Martinezhuitle, M. Rodrigo, I. Sirés & O. Scialdone, “Single and coupled electrochemical processes and reactors for the abatement of organic water pollutants: A critical review,” Chem. Rev., 115 (24), 13362-13407 (2015).
14. Y. Wang, H. Lin, F. Jin, J. Niu, J. Zhao, Y. Bi & Y. Li, “Electrocoagulation mechanism of perfluorooctanoate (PFOA) on a zinc anode: Influence of cathodes and anions,” Sci. Total Environ., 557-558, 542-550 (2016).
15. Q. Zhuo, S. Deng, B. Yang, J. Huang, B. Wang, T. Zhang & G. Yu, “Degradation of perfluorinated compounds on a boron-doped diamond electrode,” Electrochim. Acta, 77, 17-22 (2012).
16. Y. Wang, R. Pierce, H. Shi, C. Li & Q. Huang, “Electrochemical degradation of perfluoroalkyl acids by titanium suboxide anodes,” Environ. Sci.: Water Res. Technol., 6 (1), 144-152 (2020).
Past project reports
1. Introduction to Project R-122: Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 85 (6), 13 (March 2021); Full paper: http://short.pfonline.com/NASF21Mar1.
2. Quarter 1 (January-March 2021): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 85 (12), 13 (September 2021); Full paper: http://short.pfonline.com/NASF21Sep1.
3. Quarter 2 (April-June 2021): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 86 (3), 18 (December 2021); Full paper: http://short.pfonline.com/NASF21Dec2.
About the author

Qingguo (Jack) Huang is Professor in the Department of Crop and Soil Sciences, University of Georgia, Griffin Campus. He holds a B.S. in Environmental Science (1990) and a Ph.D. in Chemistry (1995) from Nanjing University, China as well as a Ph.D. in Environmental Engineering from the University of Michigan, Ann Arbor, Michigan. Dr. Huang’s research interest focuses on catalysis involved in the environmental transformation of organic pollutants, and development of catalysis-based technology for pollution control and environmental remediation and management. His laboratory has been actively involved in several cutting-edge research topics:
- Enzyme-based technology for water/wastewater treatment and soil remediation
- Electrochemical and reactive electrochemical membrane processes in wastewater treatment
- Catalysis in biofuel production and agro-ecosystem management
- Environmental fate and destructive treatment methods of PFASs
- Environmental application and implication of nanomaterials
He has published over 150 peer-reviewed journal articles, five book chapters and four patents and three patents pending. He has taught three courses at the University Georgia: Introduction to Water Quality, Environmental Measurement, and Advanced Instrumental Analysis in Environmental Studies.
* Principal Investigator (PI) Contact Information:
Qingguo Huang, Ph.D, Professor, Department of Crop and Soil Sciences, University of Georgia
1109 Experiment St.
Griffin, GA 30215, USA
Phone: 770-229-3302
Fax: 770-412-4734
E-mail: qhuang@uga.edu
RELATED CONTENT
-
Filter Press Troubleshooting and Optimization
Zachary Beckman of Haviland Enterprises Inc. discusses proper filter press maintenance for optimization of wastewater treatment systems.
-
Zinc Phosphate: Questions and Answers
Our experts share specific questions about zinc phosphate and pretreatment
-
Is Your Electroplating Waste Hazardous?
Some that bears precious metals is, and there are a host of regulations to consider when recycling.


















